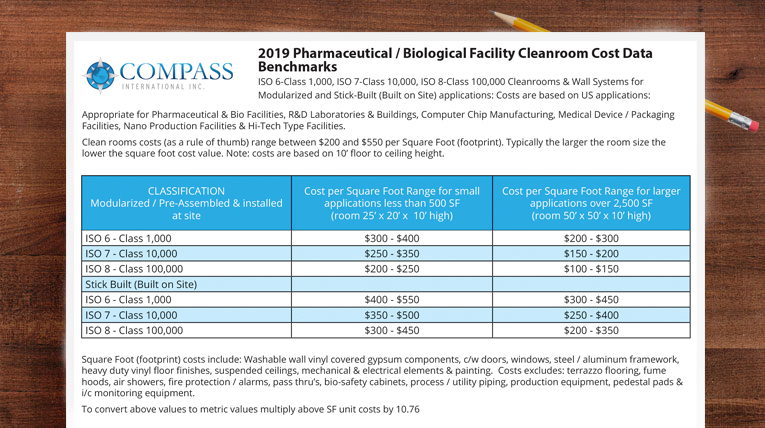
To meet requirements of a clean room as defined by federal standard 209e and newer iso standards all clean rooms must not exceed a particulate count as specified in the air cleanliness class.
Clean room classification iso vs eu.
For cleanrooms and clean zones shown in iso 14644 1 2015 cleanroom limits for airborne particulate contamination clean room and clean air device classification in relation to gmp 2008.
Grade c with class 10000 m 5 5 iso 7 and grade d with class 100000 m 6 5 iso 8.
Grades a and b correspond with class 100 m 3 5 iso 5.
In reality however you can reach an iso 6 clean room with 1 recommendation is 2 airlock.
Under the fed ste 209e system there was no equivalent for this level of cleanliness.
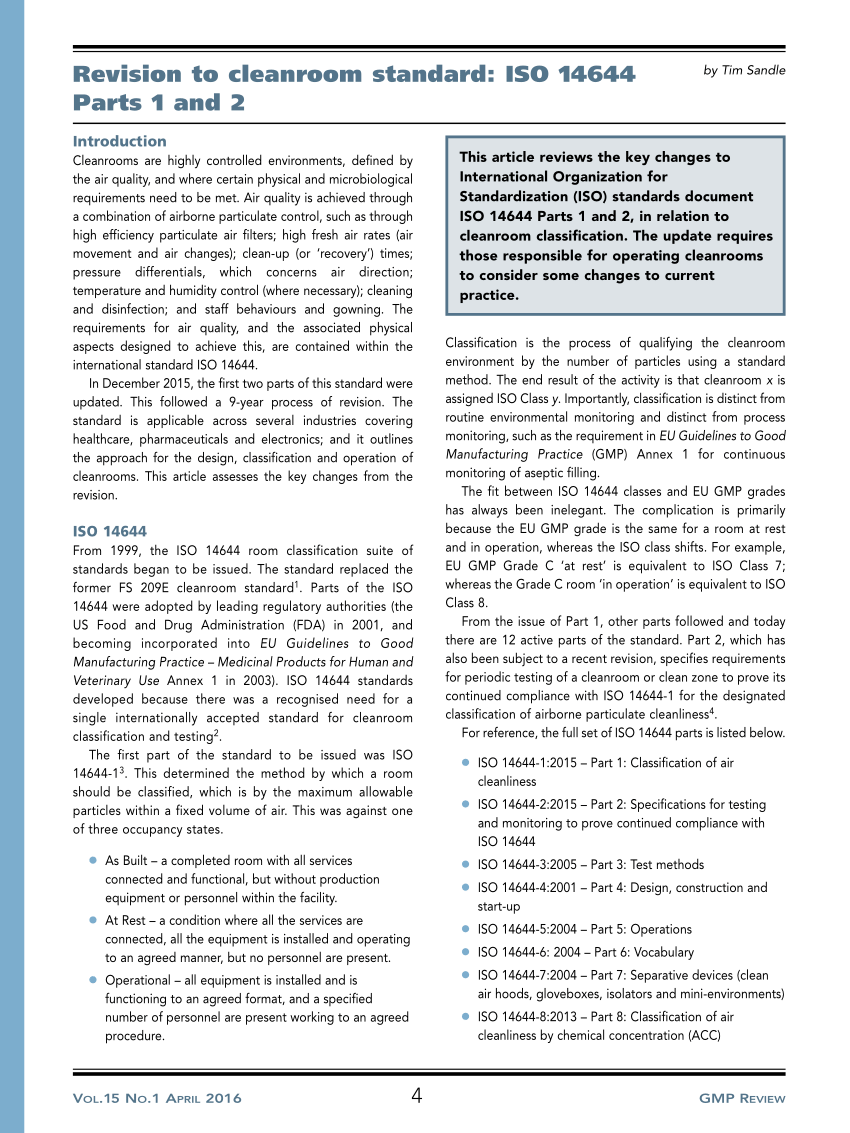
Iso 14644 1 and older standard fs 209e determine class by the concentration levels of particles.
Clean room classifications can be confusing.
B the guidance given for the maximum permitted number of particles in the at rest condition corresponds approximately to the us federal standard 209e and the iso classifications as follows.
Iso 8 is the least clean cleanroom classification.
Iso 6 cleanroom class 1 000 in theory for an entire room to reach iso 6 air cleanliness you need to enter the cleanroom via an iso 8 ante room then go through an iso 7 to finally get into the iso 6 as shown in the image.
Classification 1 is the cleanest.
Maximum permitted number of particles per m 3 equal to or greater than the tabulated size.
A cleanroom must have less than 35 200 000 particles 0 5 micron per cubic meter and 20 hepa filtered air changes per hour.
Ordinary room air is around class 1 000 000 or iso 9.
Iso 14644 1 and iso 14698.
By comparison a typical office space would be 5 10 times more dirty.
However class will greatly impact design considerations such as filtration hvac requirements and other design elements.
If you do business in europe and are installing a clean room that deals with the manufacture of sterile medicinal products your clean room must adhere to the most recent set of standards set forth in the revision of the annex to the eu guide to good manufacturing practice manufacture of sterile medicinal products.
Whereas cleanliness standards were once defined by the federal standard 209e they have been replaced and simplified by iso with classifications 1 to 9.
Iso 14644 1 and iso 14698 are non governmental standards developed by the international organization for standardization iso.
The latter to cleanrooms where biocontamination may be an issue.
The class defines a minimum cleanliness level not a specific design.
The former applies to clean rooms in general see table below.
The particles range in size from 1 5 microns µm.